Govt Warns Kenyans Against Buying & Consuming Popular Cough Syrup
The product, manufactured by a company in South Africa was recalled by the National Agency for Food and Drug Administration and Control (NAFDAC) of Nigeria.

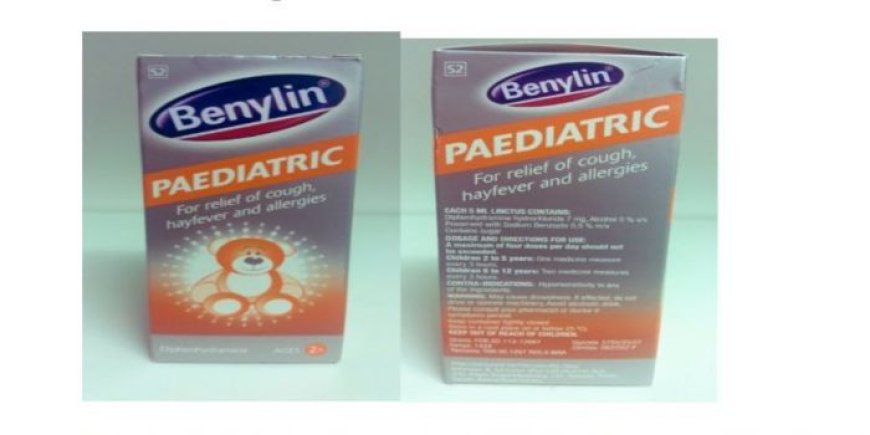
The Pharmacy and Poisons Board (PPB) has warned Kenyans against the purchase and consumption of Benylin pediatric 100mls cough syrup Batch No 329304.
PPB in a statement on Thursday, April 11 revealed that the product, manufactured by a company in South Africa was recalled by the National Agency for Food and Drug Administration and Control (NAFDAC) of Nigeria.
The board indicated that the product was being recalled due to quality concerns arising from an unacceptably high level of diethylene glycol detected through laboratory analysis conducted by NAFDAC.
Diethylene glycol is a contaminant which is toxic to humans when consumed and can prove fatal.

Pharmacy and Poisons Board offices. /FILE
Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.
"Following receipt of this information the PPB has immediately commenced investigations. The PPB also initiated rapid response, which includes sampling of batches of Benylin pediatric 100mls syrup that are within shelf life for the purpose of screening of the levels of Ethylene glycol and diethylene glycol," stated PPB in part.
"Subsequent implementation of regulatory actions will be based on the laboratory findings."
Given the above, the PPB advised all pharmaceutical outlets, healthcare facilities, healthcare workers and members of the public to immediately quarantine the product and stop distribution, sale, issuing or use of the product.
Furthermore, members of the public have been advised to return the product to the nearest healthcare facility, while healthcare facilities are advised to return the products to the respective suppliers.
However, PPB noted that the alert affected only the impacted batch of the cough syrup and not the whole drug.
"The public or patients who experience adverse reactions/events following the use of this product in children are advised to direct such patients for immediate medical attention from a qualified healthcare professional," added the board.
PPB assured the public that it has established mechanisms to ensure that medicines supplied to the Kenyan market meet the required Quality, Safety and Efficacy standards.
"We encourage the public to be vigilant at all times and report any suspected poor-quality medicines or adverse drug reactions to the nearest healthcare facility and the Pharmacy and Poisons Board," urged the board.
Benylin is a generic name for diphenhydramine, an antihistamine drug used to relieve symptoms of allergy, hay fever, and the common cold. These symptoms include rash, itching, watery eyes, itchy eyes/nose/throat, cough, runny nose, and sneezing.
It is also used to prevent and treat nausea, vomiting and dizziness caused by motion sickness.
Diphenhydramine can also be used to help you relax and fall asleep. This medication works by blocking a certain natural substance (histamine) that your body makes during an allergic reaction.