Govt Orders Quarantine Of Cancer Management Drug
The quarantine order is being issued due to a market complaint on the appearance parameter of the content.

The Pharmacy and Poisons Board on Thursday, December 5 announced an immediate quarantine of Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017.
The drug, which is manufactured by Halsted Pharma Private Limited, India, is a cancer drug that has been used in the past for injection purposes.
The quarantine order is being issued due to a market complaint on the appearance parameter of the content. Flurasted 500 (5-Fluorouracil) Injection is used in the management of cancer.
The PPB, in notifying all health practitioners and dispensing pharmacies, directed them to immediately halt the use and sale of the Flurasted 500 (5-Fluorouracil) Injection.
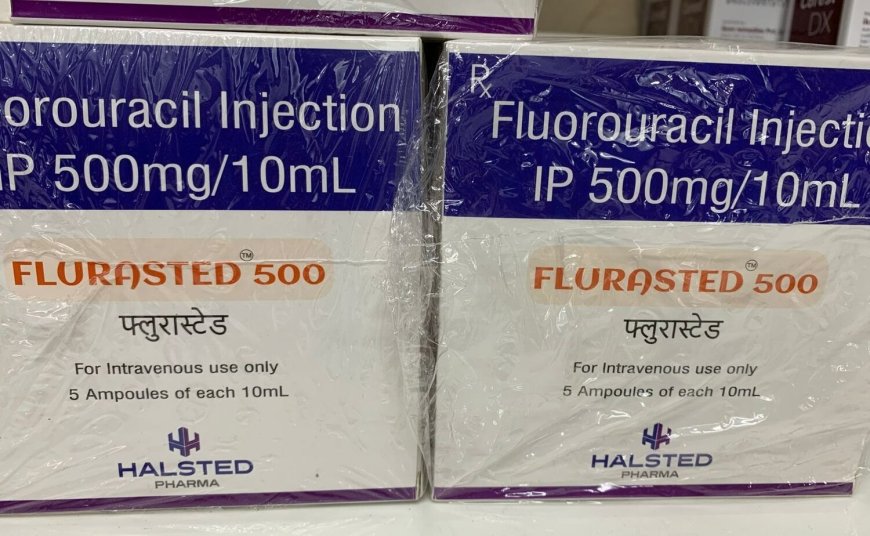
A photo of the Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017 recalled by the government on a shelf. /INDIAMART
"In light of this, the Board advises all pharmaceutical outlets, healthcare facilities, healthcare professionals, and members of the public to immediately quarantine the product batch and STOP the further distribution, sale, issuance, or use of the affected batch," stated PPB in part.
PPB CEO Fred Siyoi further encouraged the public to report any suspected cases of sub-standard medicines or adverse drug reactions to the nearest healthcare facility or the Pharmacy and Poisons Board channels.
Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017 is a cancer medication used in the treatment of various types of cancer, particularly for chemotherapy.
Additionally, the active ingredient, 5-Fluorouracil (5-FU), is a chemotherapy drug that interferes with the growth of cancer cells, and it's commonly used to treat cancers like colon, breast, and stomach cancer.
This recall comes on the backdrop of yet another one issued by the board on November 22, of two nasal drops; Efinox 1% w/v Batch No. 82979 and Efinox 0.5% w/v Batch No. 82978 manufactured by Laboratory and Allied Limited based in Kenya.
In a statement, PPB revealed that the recall was issued due to labelling mix-ups where the correct product was identified, but the wrong strength was applied.
Following this discovery, the board called on all pharmacies, medical facilities, doctors and Kenyans to cease distributing and selling the drug immediately.
Efinox is a nasal drop and is used for temporary relief of congestion in the nose caused by various conditions including the common cold, sinusitis, hay fever, and allergies. According to WebMD, it works by narrowing the blood vessels in the nose area, reducing swelling and congestion.