Govt Lifts Quarantine On Drug Used To Relieve Toothaches, Headaches
PPB explained that the decision was based on comprehensive quality control tests, which verified that the product complies with all necessary health and safety standards.

The Pharmacy and Poisons Board (PPB) has revoked the quarantine order on Mefnac Oral Suspension (Mefenamic Acid 50mg/5ml) after conducting a thorough investigation into safety concerns over several months.
PPB explained that the decision was based on comprehensive quality control tests, which verified that the product complies with all necessary health and safety standards.
"The Pharmacy and Poisons Board ("the Board") has lifted the quarantine order issued on 11th of December 2024 through a public alert for Mefnac Oral Suspension (Mefenamic Acid 50 Mg/5 Ml) Manufactured by Efroze Chemical Industries Pvt Ltd, Pakistan," announced PPB in part.
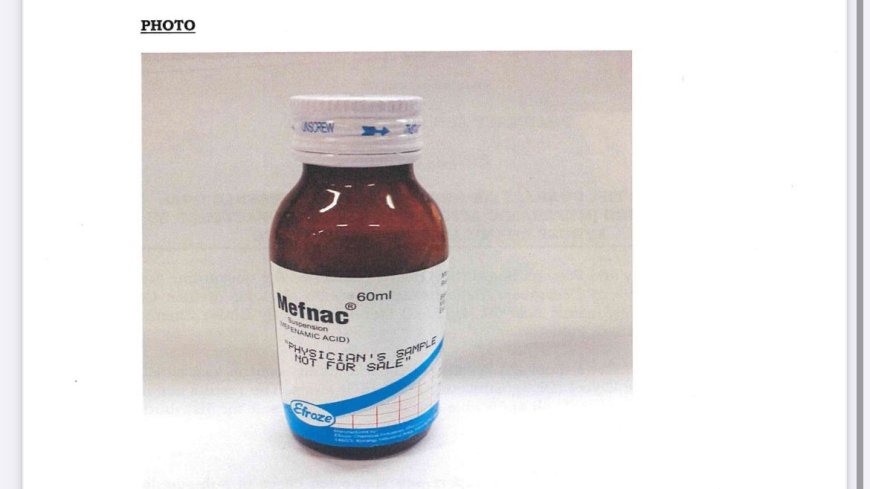
An image of Mefnac Oral Suspension. /PHARMACY AND POISONS BOARD
The board clarified that the initial quarantine, enforced on December 11, 2024, was a precautionary step taken due to concerns over possible contamination.
Laboratory tests have since confirmed that the drug is safe for distribution and use. The analysis specifically aimed to detect Diethylene Glycol (DEG) and Ethylene Glycol (EG), two substances that can cause serious health complications when consumed in large amounts.
"The decision follows thorough investigations and quality control assessments for Diethylene Glycol (DEG)/Ethylene Glycol (EG) levels. The results confirm that the product meets all applicable specifications and is safe for distribution and use," added PPB.
Mefnac Oral Suspension (Mefenamic Acid 50mg/5ml) is an anti-inflammatory medication commonly used to alleviate mild to moderate pain, such as headaches and toothaches.
Additionally, some women use Mefenamic Acid to manage severe menstrual pain (dysmenorrhea). The drug works by reducing the production of prostaglandins—hormones responsible for pain, inflammation, and fever.
Following the PPB’s latest announcement, Mefenamic Acid will once again be available in medical facilities, with the board confirming that licensed pharmacies will have access to the drug.
Meanwhile, Kenyans have been urged to stay alert against substandard medicines that may enter the market following this decision. One warning sign of poor-quality drugs, including Mefenamic Acid, is the occurrence of unexpected adverse reactions.
The Pharmacy and Poisons Board has advised patients to report any such incidents directly to them.
"The Board urges the public to report any suspected cases of sub-standard medicines or adverse drug reactions to the nearest healthcare facility or the Pharmacy and Poisons Board," advises the Board.
In December 2024, PPB announced an immediate quarantine of Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017, a cancer drug that has been used in the past for injection purposes.
The quarantine order was issued due to a market complaint on the appearance parameter of the content. Flurasted 500 (5-Fluorouracil) Injection is used in the management of cancer.